Company details for:
Biopharma Group

Biopharma House,
Winnall Valley Road,
Winchester,
Hampshire,
SO23 0LD,
United Kingdom
Quick Links:
Products / Services
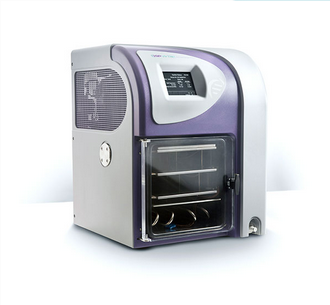
Laboratory / Benchtop Scale Freeze Dryers
Benefits offered by the Advantage Pro
Designed with both scale-up and scale-down, in mind,
Cascade refrigeration provides a -82°C large 6L capacity condenser coil, enabling lyophilisation of a wide variety of products and solvents.
Optimal heat transfer - silicone flooded shelves mean the AdVantage Pro maintains a precise level of temperature control (+/- 1.0 °C).
Fully customisable - up to ten thermal treatment and twelve drying steps for the ultimate recipe customisation.
Secure data storage and trending software option - ethernet-enabled for effortless data sharing. Developed to meet the lyophilisation requirements of today’s pharmaceutical and biotechnology applications.
Compact design and small footprint - Only 56.5 cm wide, yet with up to 2,766 cm2 of usable shelf area, the AdVantage Pro enables product to be safely processed using minimal bench space.
AdVantage Pro Freeze Dryer Trending Software
Supplied pre-loaded on a dedicated laptop PC offering plug and play ethernet connectivity to your AdVantage Pro freeze drying unit (when purchased with an AdVantage bench top unit − retrofit options are available). This iFIX-based software package provides graphics and interactions complimenting those found in the Merlin and LyoS series of Pilot scale control and trending software so simplifying upscaling from bench top processes.
What it has:-
Recipe manager with 16 recipes
Continuous historical data trending
Proficy Historian data archiving
Data export capability

Pilot & R&D Freeze Dryers
Benefits offered by the Genesis & Ultra
A perfect solution for operators seeking versatile freeze dryers capable of cross-over between applications / batches us at the pilot and small scale production, level
Flexibility - an extensive choice of powerful options and add-ons enables easy scale-up from research to full scale production.
With over 100 flexible configurations - the ideal choice whether you’re in academic research, a biotechnology or pharmaceutical company, part of a government agency, or working in industrial manufacturing.
Easy to customise -up to 6 shelves (Genesis) or 15 shelves (Ultra) for personalised configurations. Ultimate versatility with bulk or stoppering configurations, air cooled or water cooled, can accommodate both ‘wet’ and ‘dry’ vacuum pumps to meet the preference of the user
Range of options - from manual controls to the latest automated control with cGMP data monitoring and automation applications for 21 CFR Part 11 requirements
All controls and features are match those used in performance matched SP VirTis brand industrial lyophilisers, with shelves and chambers engineered using premium chemical resistant 316L stainless steel. Thereby providing advanced, reliable and scalable lyophilisation.

Aseptic Production Freeze Dryers
LyoConstellation + LyoGalaxy series of aseptic freeze dryers have been designed with throughput capacity (including automated loading / unloading), product efficacy, cleaning validation, and data integrity 21 CFR Part 11 needs, in mind. Our host of PAT technology offers an abundance of real time data to support the day-to-day operations as well as, the QP for those occasions when deviations may arise.

Food Production Freeze Dryers
Ranging from compact benchtop models to pilot and commercial scale machines, the equipment is scalable and highly customisable for a range of industries and applications.

Rotary Vial Washers
With minimal footprint, and the capability to recycle, filter and re-use WFI, the range can cover the full range of pharmaceutical vials (2-100 mL) with efficiency in mind. Each vial formal has an HMI selected “recipe”, with specific settings for a variable such as spraying time and indexing time.
Benefits
Dependably effective washing: Guaranteed reduction of particles by 1000 times and spray manifold eliminates the risk of chipping vials
Suitable for almost any manufacturing space: Small footprint and reduced water usage
Highly efficient and economical to run: Simple servodriven operation with fewer moving parts reduces maintenance and dedicated recipes, to ensure vastly reduced water usage
Extremely flexible: Easy and quick change between different sizes of glass or plastic vials

External Vial Washers
By enclosing the aluminium cap between two opposed belts, the seal that is created prevents the cleaning agent from entering the cap area, thereby eliminating potential future bacterial contamination.
Advantages include:
• High washing efficiency
• Highly effective drying
• Protection of the seals
• Flexible transport system
• Short and reliable changeover time
• Longer processing time

Sterilising Tunnels
Heat is generated by silicon controlled rectifier elements. Depending on the format vials stay inside the sterilising chamber for approximately 6-10 minutes. The recirculated hot air is blown at a speed of approximately 0.7m/s over the vials, and remains within 2°C of its setpoint. SP i-Dositecno sterilising tunnels are fully compatible with SP i-Dositecno vial handling solutions and trayloaders and can be integrated into other existing or new installations.

Trayloaders
PLC control ensures that stepper motor speed and actuator movement is specific to each vial type and size. Changeover is quick and tool-free, taking less than 2 minutes. The TL series will handle plastic and glass vials up to 500ml with speeds of up to 400 vials per minute.

Food Production Freeze Dryers
Ranging from compact benchtop models to pilot and commercial scale machines, the equipment is scalable and highly customisable for a range of industries and applications.

R&D Freeze Dryers

Pilot Freeze Dryers

Microbiological Safety Cabinets
Specifically, these Cytotoxic Drug and Microbiological Safety Cabinets are designed and built to the performance requirements of the EN 12469:2000 European Standard and DIN 12980:2005 Standard.
The cabinets feature triple filter ‘safe change’ technology: 100% of the air is filtered via the main H14 HEPA filter directly below the work surface, with 70% of the air re-circulated via the recirculating H14 HEPA filter within the cabinet, and the remaining 30% discharged through an exhaust H14 HEPA filter.

Fume Cupboards/ Hoods

HT Series Evaporators
A Solution for High Throughput Evaporation.
Genevac HT series evaporators are the ideal solution for parallel evaporation bottlenecks in high throughput and production laboratories having high performance and high sample capacities. The unique design of the multi-layer rotor ensures efficient use of valuable laboratory bench space as well as high performance and high throughput evaporation.

EZ-2 Evaporators
Solvent removal is an important step in a vast number of applications in many disciplines. The cutting-edge design of the EZ-2 delivers many advantages when it comes to solvent evaporation requirements and fitted with advanced technologies such as the Dri-Pure® anti-bumping system to prevent sample cross-contamination, automatic end of run detection, and sample temperature control software for precise temperature control. With unrivalled versatility, this ensures effortless, fast, and safe sample evaporation to give total peace of mind.

Rocket Evaporators
There are two systems available:
Rocket Synergy is designed to dry or concentrate six flasks in parallel, each containing up to 450ml of solvent. Alternatively, up to 18 ASE vials can be used. If you are running 3 or more rotary evaporators at the same time, then the Rocket Synergy is for you.
Concentrate to a defined volume
Custom methods
Very high speed
Unattended parallel operation
Concentrate directly into your vial with SampleGenie

Automation Ready Cryostorage Freezer Systems
The Revolution Series range of cryostorage freezer systems, are the latest in technology design incorporating the ability to automate sample retrieval and manage samples locally via cloud-based software.
Benefitting from extensive experience in vacuum technology by the developers, the Revolution range features extremely low LN2 consumption, complemented with a PLC-based touchscreen controller allowing modules to be added to suit customer requirements. In addition, dual liquid level measurement means that your samples are protected from over and non-filling of liquid nitrogen.

Revolution IVF - High Capacity Freezer
With an increased capacity (up to 700% more than a similar footprint), auto-fill, liquid submersion or vapor storage, leading edge security and other special features designed with embryologists in mind, this high-capacity freezer will revolutionise your IVF operation.

Labs Series Cryostorage Freezer Systems
Cryogenic storage in either liquid or vapour phase applications, make the LABS Series the right choice for long−term sample storage application, especially where temperature stability and sample security are required.

High Pressure Homogenisers

Avestin Range of Pilot Machines

Production Scale High Pressure Homogenisers

Cell Lysis & Extruder Systems

Analytical Lyo Instruments
Determining critical temperatures is fundamental to the freeze drying process. Biopharma Group specialists have designed and developed three advanced analytical instruments − the Lyostat freeze-drying microscope, the Lyotherm DTA and impedance analyser and the MicroPress specifically to aid those employing freeze drying technologies within their production or research projects as well as being involved in the development of a compatible DSC imaging stage.
A rational, knowledge-based approach to designing and developing successful freeze drying formulations and cycles requires information about how the product responds to different processing conditions. Candidate formulations should be analyzed in terms of their visible physical structure, thermal characteristics and frozen state mobility, in order to gain a sufficient understanding of its behaviour throughout the freeze drying process. This ensures that cycles can be developed that are tailored to the specific needs of every product, making them efficient, safe, robust and reproducible.
With each product and process recognised as unique, by entrusting us with your project, you will benefit from our depth of experience and knowledge to ultimately reach any project goal. Each project will be carefully managed by Biopharma Group’s team of scientists and consultants. Through the operation of proprietary and advanced equipment, both R&D and manufacturing activities will run efficiently.
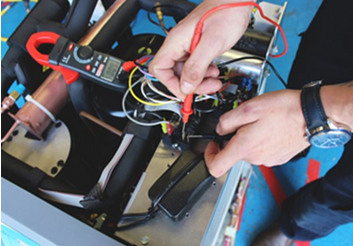
Technical Service & Maintenance
About us
At Biopharma, our goal is to furnish you with the appropriate equipment and extend any necessary advice and support. With extensive experience in the processing industry and profound understanding of our supplied equipment, we are well-equipped to assist you. Our seasoned service department maintains robust relationships with our suppliers, ensuring comprehensive support throughout the lifespan of your equipment. Additionally, our in-house freeze drying laboratory provides a comprehensive array of services for product and process development.
Biopharma Technology Ltd. offers specialised contract research, analysis, and development services, along with training and advanced instrumentation for the worldwide biopharmaceutical and associated sectors. They possess exceptional expertise in all facets of freeze drying technology.
Our Equipment Ranges Include:
- Freeze Dryers
- Vial Handling & Fill Finish Equipment
- Food Production Freeze Dryers
- Faster Airflow Equipment
- Centrigugal Solvent Evaporation
- Cryopreservation Storage Equipment
- High Pressure Homogenisers
- Analytical Lyo Instruments
Our service department offers comprehensive technical support for all our freeze dryers. With strong connections to our suppliers and a vast installed base exceeding 400 freeze dryers of various sizes and setups, you can trust in our expertise. Alternatively, if you have your own service engineers, we can provide training in equipment care and maintenance. We have invested in top-notch calibration equipment and can conduct on-site calibration of all instruments to UKAS standards.
The Biopharma Group consists of various divisions tailored to specific regions and requirements. Our goal is to tailor our services to meet the unique requirements of our customers' projects, regardless of their size or stage. Whether they require equipment purchase, a single cycle run/analysis, or a comprehensive formulation development program, we aim to complement their in-house expertise effectively.
Equipment Sales & Technical Service: Being a top equipment provider for the pharmaceutical, biotech, and process sectors across the UK, Ireland, and France, we offer extensive experience in processing industries. Our specialised product lineup encompasses pharmaceutical and food production freeze dryers, centrifugal solvent evaporators, microbiological safety cabinets, aseptic processing lines and fill-finish solutions, cryopreservation storage solutions, and analytical instruments.
Contract R&D & Manufacturing Services: Established in 1997, our Contract Development and Manufacturing Organisation (CDMO) division was created to offer impartial contract research, analysis, and development services, lyophilisation training, and analytical instrumentation on a global scale. We provide exceptionally comprehensive services and training courses, which can be scheduled or tailored to meet specific needs, covering every facet of freeze drying technology from pre-formulation to production and dried product analysis. Additionally, we continue to lead in the development of analytical instrumentation, with recent launches including the Lyostat5, Lyotherm3, and MicroPress.
Biopharma Technologies France (BTF): BTF provides a comprehensive package of equipment sales and expertise drawn from our CDMO division. This complete solution is tailored to meet the needs of our French-speaking clientele.
We provide an exceptionally thorough service and training programs that encompass every aspect of freeze-drying, spanning from pre-formulation to full-scale production and analysis of dried products.
Biopharma Group is dedicated to delivering exceptional service to our customers. We strive to maintain controlled, safe, and dependable practices for the benefit of our staff, customers, and the products we handle. To this end, we have implemented a Quality Management System that upholds the highest standards in our laboratory, documentation, and health and safety practices. Our adherence to the UKAS Management Systems guarantees compliance with ISO 9001:2015 standards.
Images










































Articles / Press Releases
Brochures
Reviews
Trade Associations


